22+ Calculate Entropy Change
Web Total starting entropy 186 2 205 596 J K -1 mol -1 You ended up with 1 mole of carbon dioxide and two moles of liquid water. Web How to calculate entropy changes - exam question practice.
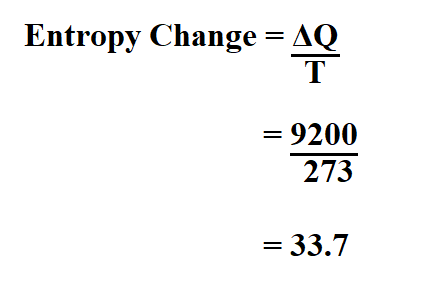
How To Calculate Entropy Change
No heat is lost to the surroundings and there.

. Web To calculate a change in entropy the initial and final values of the entropy for the system must be known. If the difference is taken between these two values the. Web The equation to calculate the standard entropy change of a system is.
Web When you evaluate the change in entropy of an entity in this case the surroundings between an initial and final equilibrium state you separate that entity from. Therefore It is because from. Web Calculate the entropy change for 100 mol of an ideal gas expanding isothermally from a volume of 244 L to 488 L.
Solution Recognizing that this is an. Web The Entropy Change of a thermodynamic system is represented as ΔS. The data about the initial and final states would suffice to define change in entropy.
The standard molar entropy of a substance is the absolute entropy of 1 mole of the substance in the standard state. Web To calculate entropy changes for a chemical reaction We have seen that the energy given off or absorbed by a reaction and monitored by noting the change in. ΔSsystemꝋ ΣΔSproductsꝋ - ΣΔSreactantsꝋ where Σ sum of For example the standard entropy.
Web Entropy changes can be calculated using the products minus reactants rule or from a combination of heat capacity measurements and measured values of. Web The entropy change for the process H 2 O s H 2 O l is 221 JK and requires that the surroundings transfer 600 kJ of heat to the system. So if say you.
Web I have a 50 mathrmg ice cube at -15 mathrmC that is in a container of 200 mathrmg of water at 25 mathrmC. We can calculate the Entropy Change of a chemical reaction or a system by using the. Total entropy at the end 214 2 699.
That means that if you are calculating entropy change you must multiply the enthalpy change value by 1000. For any chemical reaction the standard. Web This example problem demonstrates how to calculate the change in entropy of a systems and surroundings following a chemical reaction at constant.
Web Since entropy is a state function the entropy change of a system in going from volume V1 to V2 by any path will same as that of a reversible change. Crunch Chemistry 733 subscribers Subscribe 7 Share 376 views 1 year ago In part 1 of this video I walk you. Entropy is a state variable ie.
Web The change in entropy of the surroundings after a chemical reaction at constant pressure and temperature can be expressed by the formula ΔS surr -ΔHT. Web Calculating entropy change. Web But entropy change is quoted in energy units of J.
Cryptocurrencies Springerlink

Example 18 1 Predicting The Sign Of Entropy Change Ppt Video Online Download
How To Calculate Entropy Change Sciencing
Entropy

Calculating Entropy Changes 5 2 2 Cie A Level Chemistry Revision Notes 2022 Save My Exams
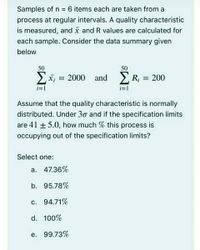
Answered Assume That The Quality Characteristic Bartleby

Problems And Solutions In Mechanical Engineering

How To Calculate Entropy Change Sciencing
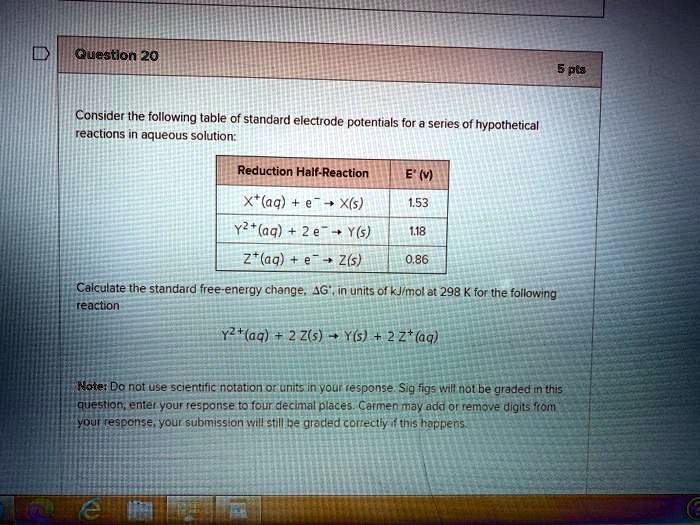
Solved Questlon 20 Pts Consider The Following Table Of Standard Electrode Potentials For Series Af Hypothetical Reactions Aqueous Solution Reduction Half Reaction E V Aq X S Y2 Aq 2 E Y S Z C Aa

Ab A2 And B2 Are Diatomic Molecules If The Bond Enthalpies Of A2 Ab And B2 Are In The Ratio 1 1 0 5 And Enthalpy Of Formation Of Ab From
Entropy

15 3 3 Calculate The Standard Entropy Change For A Reaction Hl Youtube
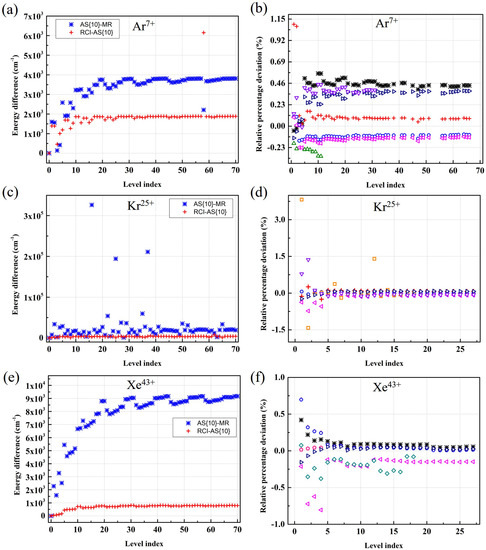
Atoms Free Full Text Extended Calculations Of Atomic Structure Parameters For Na Like Ar Kr And Xe Ions Using Relativistic Mcdhf And Mbpt Methods

Dynamics Of Shock Waves In Elastic Plastic Solids Topic Of Research Paper In Mathematics Download Scholarly Article Pdf And Read For Free On Cyberleninka Open Science Hub
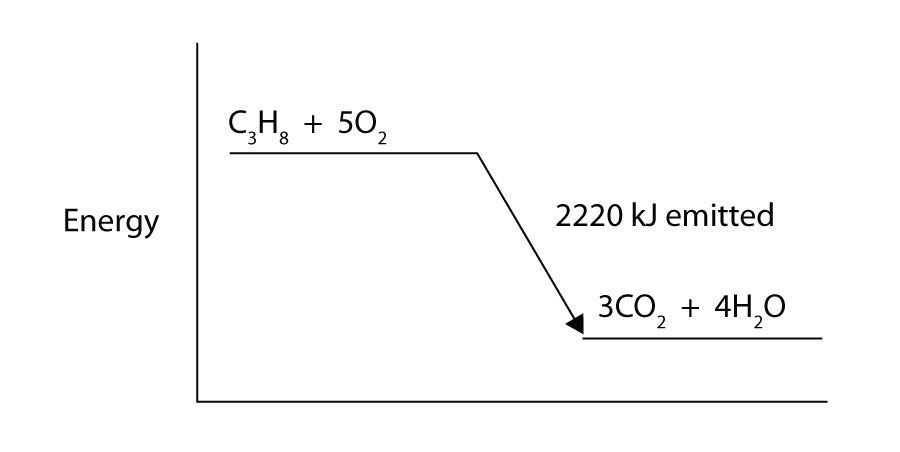
Hybrid Chem 51 V1

Pdf Std12 Chem Em 1 Pdf Sathish B Academia Edu
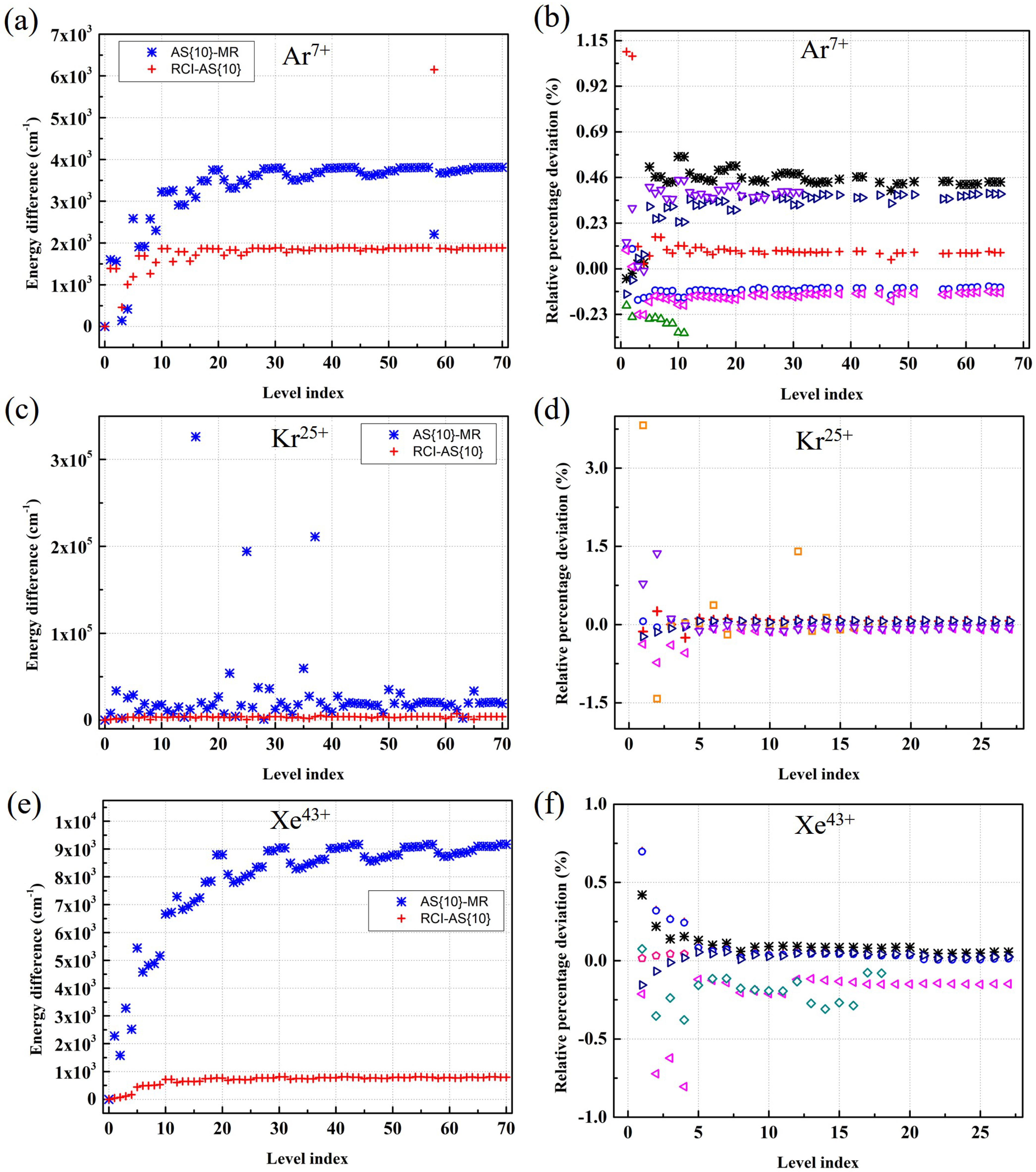
Atoms Free Full Text Extended Calculations Of Atomic Structure Parameters For Na Like Ar Kr And Xe Ions Using Relativistic Mcdhf And Mbpt Methods